Determination of Radiochemical Purity of 99mTc-DTPA Using One-System Method of Paper Chromatography
Abstract
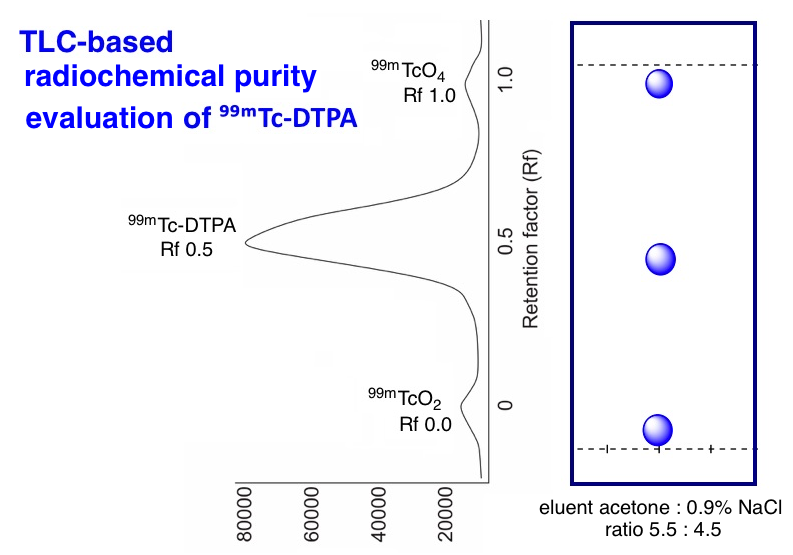
The more efficient and effective quality control techniques for 99mTc-DTPA are needed because 99mTc has a short half-life of around 6.0 hours. We have succeeded in developing a one-system of TLC for radiochemical purity testing system that is faster and more practical. Two-system method of TLC for radiochemical purity testing uses mobile phase of methyl ethyl ketone as an A and 0.9% sodium chloride solution as a B. One-system method uses the mobile phase of a mixture solution of acetone and 0.9% sodium chloride. In this study, the determination of radiochemical purity of the one-system of TLC has been successfully developed using the Whatman-1 paper stationary phase and the mixed mobile phase between acetone and 0.9% sodium chloride solution. The mobile phase of acetone: 0.9% sodium chloride with a ratio of 9:1 shows the most optimum results. This phase can separate 99mTc-DTPA (Rf = 0.4-0.6) from 99mTcO4- (Rf = 0.9-1.0) and 99mTcO2 (Rf = 0.0-0.1) as radiochemical impurities. This result shows that the one-system of TLC method can be used for radiochemical purity testing of 99mTc-DTPA radiopharmaceutical kits. This method can completely separate the product compound (99mTc-DTPA) from its impurities (99mTcO2 and 99mTcO4-).
References
[1] Fitri Imelda, Endang Susalit, M Bonar M Marbun, C. M. R., J. Penyakit Dalam Indonesia, 2017, 4, 128–136.
[2] Maskur, Purwoko, Chairuman, Yono S., Pros. Semin. Nas. Kim. FMIPA Univ. Negeri Surabaya, 2015, 11–18.
[3] Stefanus Santosa, Agus Widjanarko, C. S., J. Pseudocode III, 2016, 163–170.
[4] Sevin Ayaz. J. Clin. Anal. Med., 2017, 8, 8–11.
[5] Abdel, M. & Hamed, G. Iran. J. Nucl. Med., 2017, 25 (1), 17–22.
[6] Piepsz, A., Eur. J. Radiol., 2002, 43, 146–153 (2002).
[7] Delima, E. T. et al. Bul. Penelit. Kesehat., 2014, 45, 17–26.
[8] Levey, A. S. & Coresh, J. Lancet, 2012, 379, 165–180.
[9] Wiguno Prodjosudjadi, A. S., J. Ethn. Dis., 2009, 19, 33–36.
[10] Williams, J.K.Y., Nurs. Clin. NA, 2017, 52, 575–587.
[11] Serdar Savas SGul, Zekiye Hasbek, Taner Erselcan, Melih Akyol, B. T. Acta Medica Mediter., 2015, 31, 1247–1252.
[12] Kim, Y., Ha, S., So, Y. & Lee, W. W. Eur Radiol., 2014, 24, 413–422.
[13] Razaq, T., Nisar, H., Roohi, S., Shehzad, A. & Ahmad, I. Iran J Nucl Med, 2017, 25, 122–128.
[14] Taylor, A. T. J. Nucl. Med., 2014, 55, 1–9.
[15] Maskur, Enny Lestari, Endang Sarmini, Yayan Tahyan, Amal Rezka Putra, Dede Kurniasih, A. H. G. Urania, 2017, 23, 57–68.
[16] Sadeghpour, H., Alavi, M., Shahedi, M., Entezarmahdi, S. M. & Sakhteman, A., Trends Pharm. Sci., 2015, 1, 15–19.
[17] Shankar Vallabhajosula, Ronan P. Killeen, and J. R. O. YSNUC, 2010, 40, 220–241.
[18] Zimmerman, B. E., Herbst, C., Norenberg, J. P. & Woods, M. J. Appl. Radiat. Isot., 2006, 64, 1142–1146.
[19] Millar, A. M., Brien, Ã. L. M. O., Beattie, L. A. & Craig, F. J. Label Compd. Radiopharm., 2010, 53, 11–14.
[20] Leonardi, N. M., Casale, G. A., Nicolini, J., Zubata, P. D. & Zubillaga, M. B. J. Nucl. Med. Technol., 2015, 40, 271–275.
[21] Candela, M., Tesán, F. C., Leonardi, N. M., Zubillaga, M. B. & Salgueiro, M. J. Appl. Radiat. Isot., 2013, 82, 322–324.
[22] Korkmaz, M. & Özer, A. Y. J. Pharm. Sci., 1996, 22, 105–110.
[23] Maioli, C., Lucignani, G., Strinchini, A., Tagliabue, L. & Del Sole, A. Acta Biomed., 2017, 88, 49–56.
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.







